Fire dynamics
By Lance Bushie
Features Fire Ground TrainingFirefighters must understand what heat is and different heat phenomenon like heat flux and heat release rates to understand how to stay safe. In the past five years, new research has changed how firefighters think about fire growth, spread and extinguishment.
 Firefighters must understand what heat is and different heat phenomenon like heat flux
Firefighters must understand what heat is and different heat phenomenon like heat fluxFire behaviour has changed in the past 30 years and our tactics have not kept up with the changes in fuels and building construction. Today’s fires are far more dangerous and can exceed the limitations of our PPE. These conditions necessitate that all firefighters gain a deeper understanding of fire dynamics — how fires start, spread and develop.
First, we must understand heat and how it interacts with our structural fire fighting ensemble. We begin by understanding that all objects are in motion. The amount of motion or the vibration of the molecules colliding is energy. Heat is not temperature. Temperature is the measure of the energy contained in the object representing the speed or vibration of particles. A thermometer represents a scale of how fast those molecules are moving. The measurement on the thermometer is the equilibrium of the surrounding vibrating particles and the fluid in the glass tube. Heat is the transfer of energy from one object of high energy to an object of lower energy. The transfer of heat occurs in three ways: radiation, conduction and convection.
Radiation is electromagnetic energy emitted from the heated gases (smoke layer) and heated surfaces (walls and furniture) in all directions at the speed of light to the point of absorption. Our protective ensemble absorbs the radiation, causing the molecules in the material to vibrate faster.
Conduction is the transfer of energy from molecule to molecule by direct contact. The warmer or faster vibrating molecules cause the cooler, slower molecules to speed up and vibrate faster. As an example, impingement of a flame on a pipe transfers the energy through the pipe from hot to cold and heats up the materials on the other side of the wall where the pipe exits the wall, causing a fire in the adjoining room. Your glove, for example, makes contact with a hot wall and the higher energy (heat) in the wall is in directly transmitted to the lower energy (cooler) material of your glove. If your glove continues to absorb energy, the glove material will become a higher energy than your skin inside the glove, resulting in the energy transferring again now from the glove to your skin.
Convection is the transfer of energy through liquid or air. As the gases in a fire gain energy and warm up, they expand and become less dense as compared to the cooler room air. Currents form as the super-heated gases rise, creating a positive pressure and the fire, like a pump on an engine, creates a low pressure sucking in cool dense air from the room or outside the room. Convection occurs, and the moving gas layers create what’s known as a flow path. A flow path allows fresh air to feed the fire, possibly from an open door to the outside air. The fire also needs an exhaust for the super-heated fire gases to escape. This can occur in the same opening, like a door, creating a bidirectional flow path. If firefighters are advancing on the fire through this bidirectional flow path, hot gases will be passing overhead. If the superheated gases are in contact with structural firefighting ensemble, energy will be transferred very efficiently way from the gases to the firefighter’s PPE. This is also referred to as heat flux expressed in area of surface and time of exposure. A hot day with no humidly is equivalent to 1 kW/m2 of heat flux. At 5kw/m2 of heat flux, pain to the skin occurs in seconds. Twenty kW/m2 of heat flux is experienced at floor level during rollover. Eighty-four kW/m2 is the Thermal Protective Test as per NFPA 1971. Heat flux of 60-200 kW/m2 is a flame front across a surface or flashover. A heat flux of 20 kW/m2 can be encountered in super-heated gases within the exhaust flow path and a pretext to flashover. In simple terms, stay low and do not expose yourself to the flow path of gases exiting the structure. Also, cool as you advance.
Next, we must understand the amount of energy released from the fuels involved in the fire. Heat Release Rate (HRR) is the single most important variable in a fire. HRR is the rate at which the energy is generated by burning and the power (energy over time) released by fire, which is expressed as joules per second or watts. To simplify, it is the speed at which energy stored in an object is released under burning conditions. Take one candle burning at a temperature of 1500 F/816 C and 80 watts of energy. Combine 10 candles and the temperature remains constant but the amount of energy released multiplies by 10 to equal 800 watts. As an example, burn the same volume of wood as compared to styrene (styrofoam cup). Styrene is a good example of the furniture, flooring and wall coverings in modern buildings. Most modern furniture is comprised of petroleum or natural gas-based materials; your couch is a 45-gallon drum of gasoline. Apply a flame to both the styrene and the wood and record the time it takes to completely burn. Also note the amount of smoke and soot generated by each. Spoiler alert: do this experiment outside as the styrene will burn fast and the amount of smoke is much greater compared to the wood. What you’ll have observed is the difference in heat release rates. The wood takes more energy to begin to pyrolyze, or generate the amount of heat required to turn the solid wood to a gas. Wood will also release the gases (fuel) slower than the styrene. The styrene’s HRR is much higher than the wood. This starts to explain why today’s room contents are far more dangerous to firefighters. The amount of energy from an upholstered chair has enough stored energy at 2 megawatts (2 MW) to flashover a room and the chair will burn far more rapidly than the legacy solid wood and cotton fibres because of its high HRR.
So, how does this relate to our superhuman suit? Here are some benchmarks to remember for bunker gear: Ordinary conditions like overhaul are done in 68F/20C to 158F/70C with a heat flux of 1 to 2 kW/m2. Routine conditions like interior fire attack are 158F/70C to 200C with a heat flux of 2 to 15 kW/m2. Emergency conditions are those that are encountered in rapid and dangerous fire behaviours and have temperatures of 392F/200C and above with 15 to 200 kW/m2 of heat flux.
Our bunker gear is tested to keep heat from reaching our skin, but what’s not taught is that heat is much like nuclear energy. We must think in terms of time, distance and shielding. Our PPE can only withstand a finite amount of thermal energy transfer in terms of time. With greater amounts of heat flux, the time it takes the energy to penetrate and saturate the layers of your PPE decreases. In relation to flashover temperatures (1100F/593C and above), we have seconds. At 600F/316C we have approximately 15 seconds. At 500F/260C with moderate heat flux values of 5 to 10 kw/m2 we have 10 to 15 minutes. In terms of distance, the further from the heat we are, the less transfer of energy occurs. Using the reach of our fire streams to cool surroundings and maximize the distance between ourselves and the heat will increase the time we have to operate in the fire environment. Shielding is the next defense from heat. When approaching the fire room use the walls to limit exposure to you and your gear. Use any natural barriers between you and the fire.
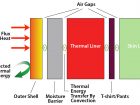
This brings us to the construction of our bunker gear, or your shield, our main line of defence from thermal energy. It is comprised of three layers: the outer shell, the moisture barrier and the thermal liner. Between each layer is an air gap. Include the station wear or shirt and pants and we have four layers with four air gaps. Heat acts on the outer shell by conduction. Once the heat has absorbed through the outer shell it slows and the air gap begins to warm via convection. Then the air gap conducts heat to the moisture barrier. This process repeats through the thermal liner and your shirt till the heat is now acting on your skin and you feel uncomfortable or you begin to feel “bee stings”. This process can be sped up if your gear is incorrectly fitted and the air gaps are limited. When bending knees, elbows or the air pack is compressing the air gaps, the heat transfer occurs five times faster as the layers are compressed, and conduction is more efficient without the air gaps. Don’t slap your buddy on the shoulder as a job well done — it can burn. Wearing coveralls in an addition to the above can give you more time by providing an extra layer for conduction and convection. It might be the difference of first or second degree burns or provide that few extra seconds from incapacitating heat and making it to the exit.
As bunker gear is designed to absorb heat, once you notice the heat coming through the gear to your skin you don’t have much time left to react. Flashover temperatures and heat flux rates rise faster than you can react. If your gear has absorbed significant amount of thermal energy during the entry and cooling of the environment has not occurred, the amount of protection your gear can provide becomes limited as it has absorbed all that it can. Constantly cool your environment, use the flow path to your advantage and be aware of the signs of flashover — free burning fire, heavy dense smoke, high heat and rollover.
NFPA 1971 requires bunker gear to pass a test exposing the ensemble layers to 84 kW/m2. Bunker gear is one piece of the ensemble of PPE we wear. The lens on our SCBA masks doesn’t require the same level of testing as our bunker gear. Polycarbonate lenses absorb certain wavelengths of light warming them above the heat encountered. Radiant heat can damage the lenses at low temperatures. Heat flux of 20 kW/m2 and a temp of 500F/260C for 2.5 min has resulted in catastrophic failure of SCBA masks. Note that those are temperatures and heat flux ranges that your bunker gear is designed to protect you from for longer than five minutes. Also recognize these are temperatures and heat flux rates that can occur in the exhaust flow path. Only since the 2013 edition of NFPA 1981 SCBA, has a radiative flux test been added of 15 kW/m2 for five minutes for the masks.
Even more prone to failure is the radios that we employ on the fireground. Testing by National Institute for Standards and Technology (NIST) has shown that radios can shut down, degrade performance or fail at temperatures as low as 212F/100C to 500F/260C. Mic cords have melted at 300F/149C and expose wires that will short and act as if the transmit button was depressed. This has occurred on fire scenes locking up the frequency. Imagine calling a mayday but the incident command is paralyzed as the channel is locked up and unable to quickly organize a response. Wear a radio harness to under the bunker jacket with just the mic exposed. This protects the weakest part of the radio and allows for better transmission of radio waves.
There have also been a number of helmets fail in fires. A good telltale indicator is your face shield lens. It has the lowest melting point of your ensemble. If you have the shield down over your SCBA mask it will absorb the thermal energy and provide some protection to your mask lens. It will also be a visual indicator you’re absorbing too much thermal energy and it’s time to leave. European fire fighting helmets have a higher heat resistance. I use Dräger helmets while teaching in the flashover chamber. When I first started teaching the flashover training as the lead instructor, we used our issued fire helmets and found that they only were able to last a couple burns in the chamber before they deformed from the amount of radiant thermal energy the instructor absorbs over a 25 min burn. We switched to the European style and have been using the same helmets for years with no thermal degradation. It you want to take the protection a level higher you can order the gold coated face shield that will reflect almost all the radiated energy. The Dräger face shield will actually cover the entire mask lens.
Be situationally aware of your surroundings. Use the tools we have available like a thermal imaging camera. This can be a great tool or be very misleading in relation to thermal temperatures. To fully understand thermal imagining takes much training and each manufacturer has a different processor resulting in different readings. I recommend basic training and using the thermal imaging cameras (TIC) as a tool that indicates changes. Use the TIC to see if your environment is being cooled effectively. Track the thermal energy changes and use the TIC to lead the attack team to the seat of the fire for extinguishment. How do we defend ourselves from thermal energy? Time, distance, shielding and a greater understanding of fire dynamics.
Lance Bushie is the lead instructor at Trident Fire Training & Consultancy Inc. www.tridentfire.net
Print this page